Download this guidance on Pharmaceutical Stability Study with SAP S/4HANA part 1/2
Guidance Vietnamese Version.pdf
Table of contents
1. Create Inspection Plan for 2 conditions: QP01
2. Create test schedule for 2 Inspection Plan: CWBQM
3. Create Batches for Stability study (With Product don’t have available in SAP): MSC1N
4. Create Notification for stability study (Protocol): QM01
5. Create/ change Initial Sample (Sampling plan): QM02
6. Confirm Initial Sample (Sample Receiving): QM02
7. Initial Test for Stability sample: QM02
8. Record Testing Results of Initial sample: QA32
9. Complete Initial Test: QM02
10. Specify Storage Conditions: QM02
11. Create/ change Testing schedule: QM02
12. Store stability samples: QM02
13. Complete Test Scheduling: QM02
14. Define Start Date of Study: QM02
15. Maintenance Plan (testing schedule): IP 24
16. Edit Maintenance Plan: IP10
17. Call the Tests for stability study: IP30
18. Record the testing results: QA32
19. Show Stability study table result: QM02
20. Complete stability study: QM02
1. Create Inspection Plan for 2 conditions: QP01
=> If the Material doesn’t have Inspection Plan: => Create New Inspection Plans: Use Transaction Code (Tcode): QP01
=> If the Material has Inspection Plan: Use Copy model in Tcode QP01. See the below guidance:
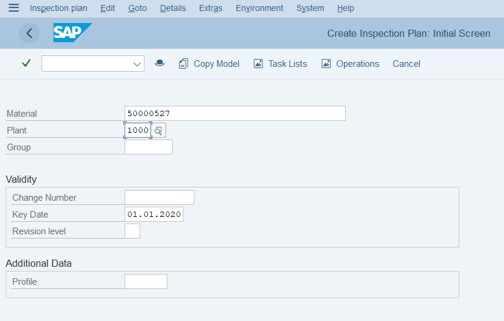
Step 1: Access Tcode: QP01
Step 2: Fill Material code, plant and Key date of New Inspection plan. (Notice: Key date should choose the date that is before the start date of stability of study).
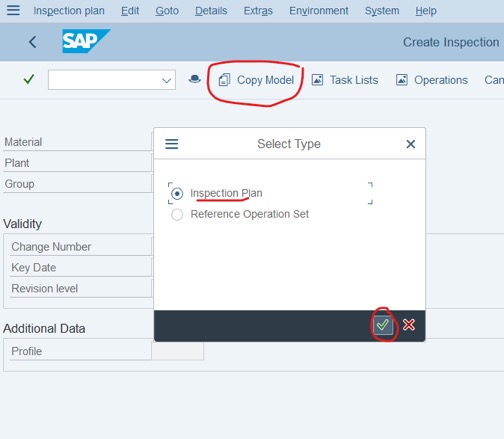
Step 3: Click Copy Model > Choose Inspection Plan > Click on V icon.
Step 4: Fill the information of the Material that has Inspection Plan (the same Material)
– Material
– Plant
– Key date
Click on V icon or Enter
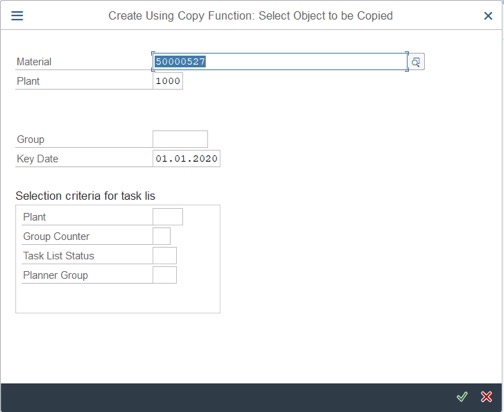
Step 5: Double click on the Inspection Plan that you want to copy.
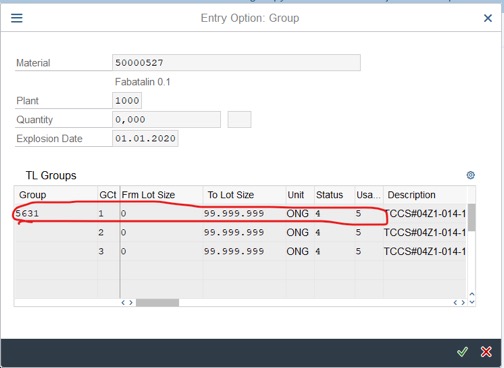
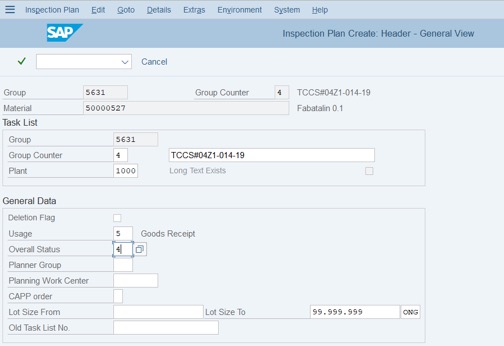
Step 6: Change Overall Status to 4 – Effective and press Enter.
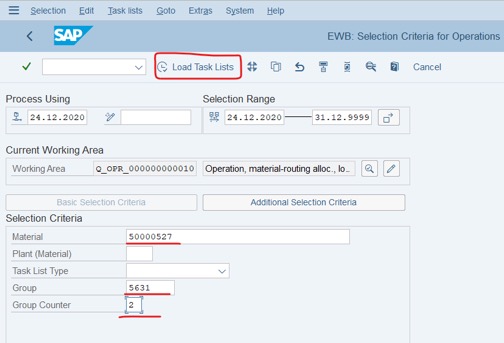
Record or remember Group and Group Counter to create a schedule. As the below picture: 5631 and 4.
Step 7: Change Description to suitable studies: Accelerated or long-term conditions. Tick on the first row => click More>> choose Inspection Characteristic Overview
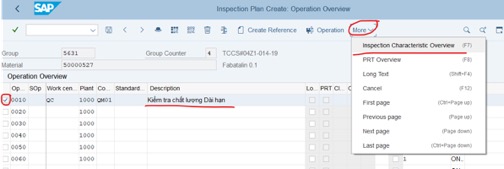
Step 8: Delete or add criterias for Inspection plan that is used for stability study:
Delete: Tick on the row that you want to delete, then click on delete icon
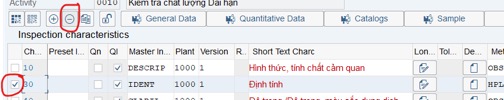
Add more criterias in the last row.
Step 9: Re-check Long text and Limit of all criterias (Sometimes when you copy Inspection Plan: Long text can be incorrect).
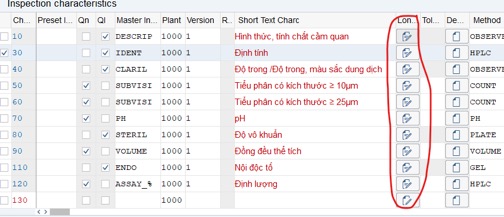
Step 10: Save Inspection Plan:
Copy Inspection Plan for the other condition.
After copy completely: We will have 1 Group and 2 Group Counter for 2 conditions.
2. Create test schedule for 2 Inspection Plan: CWBQM
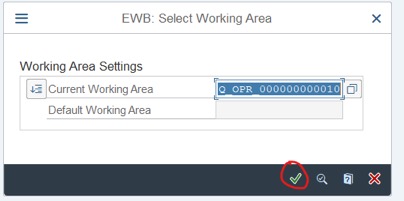
Step 1: Access Tcode: CWBQM Choose Current Working Area is : Q_OPR_000000000010 >> click on V ico or press Enter.
Step 2: In Tab: Basic Selection Criteria: Fill Material, Group và Group Counter >> Click Load Task Lists. (Group and Group Counter is created in the above step)
Step 3: Click on Task Lists >> choose Header
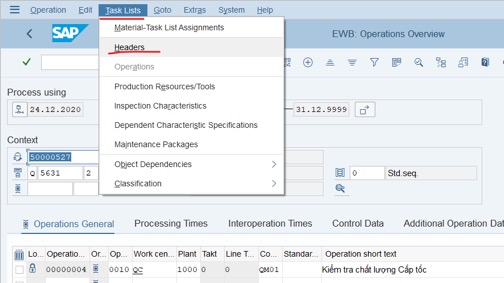
Step 4: Tick on the first row >> Click on pencil icon
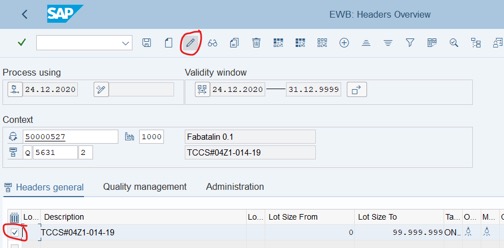
Step 5: Go to the right side of the table: Strategy column: QSTABI, StrategDescrip column. Auto change.
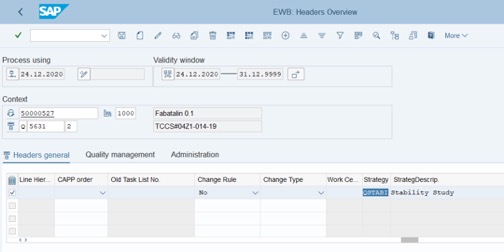
Step 6: Click Task Lists >> Choose Maintenance Packages.
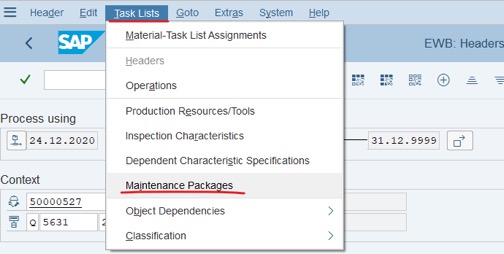
Step 7: Assign test schedule: Fill number of months into Package column: fill 01 >> press Enter, fill 03>>press Enter and fill 06 >>press Enter. Other columns will be filled automatically. After that, Click Save to complete:
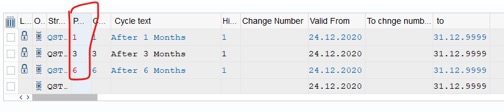
Create test schedule for the other Inspection Plan.
3. Create Batches for Stability study (With Product don’t have available in SAP): MSC1N
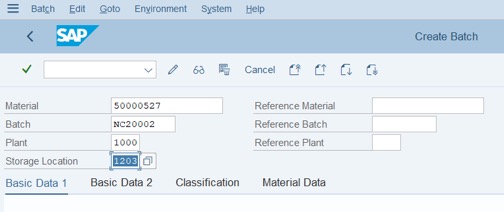
Step 1: Access Tcode: MSC1N. Fill Batch and Storage Location >> Press Enter
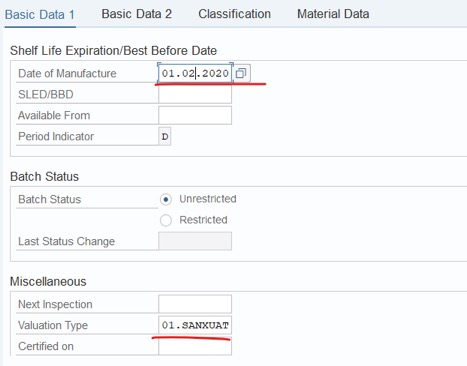
Step 2: Fill Valuation Type: 01.SANXUAT. Fill Date of Manufacture, If needed. Then Save:
4. Create Notification for stability study (Protocol): QM01
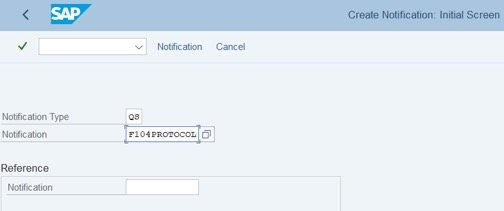
Step 1: Access Tcode: QM01.
Fill Notification Type: QS.
Fill Notification: This is the code of Protocol.
Step 2: Press Enter. Fill Material and Plant of Mat. >> Press Enter
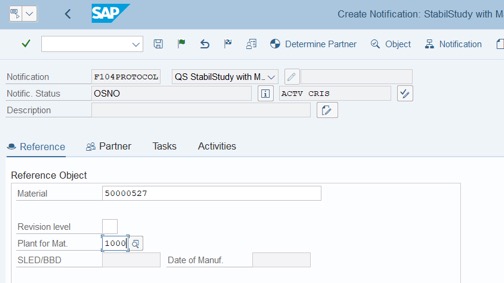
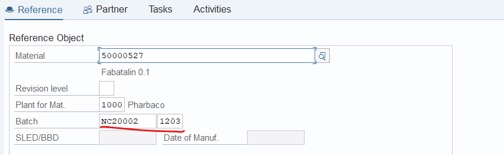
Step 3: Fill Batch Number and Storage Location. Click on Save:
Please remember this Notification code:
5. Create/ change Initial Sample (Sampling plan): QM02
Step 1: Access Tcode: QM02. Fill Notification code>> Press Enter or click on Notification.
Step 2: Click on Create Initial Sample at Action Box (the right window side).
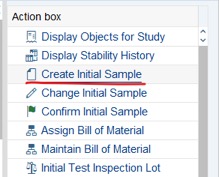
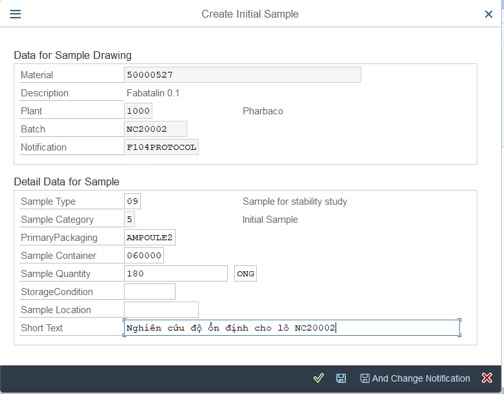
Step 3: Fill Information into tab: Detail Data for Sample
– Sample Type: Default is 09
– Sample Category: Default is 5
– Primary Packaging:
– Sample Container:
– Sample Quantity: Total sample quantity for stability study (accelerated and long-term conditions) and Unit.
– Storage Condition: If store in 2 condition, this one will be empty
– Sample Location: Fill location or empty.
– Short Text: describe this stability in short text.
Then click on Save and Change Notification:
If click on Save And Change Notification, it will stay at the working windows of Tcode QM02. If click on Save, it will save and exit.
Change initial sample: click on Its row at Action Box
6. Confirm Initial Sample (Sample Receiving): QM02
Step 1: Click on Confirm Initial Sample, Fill the Date of Receipt and click on Save And Change Notification
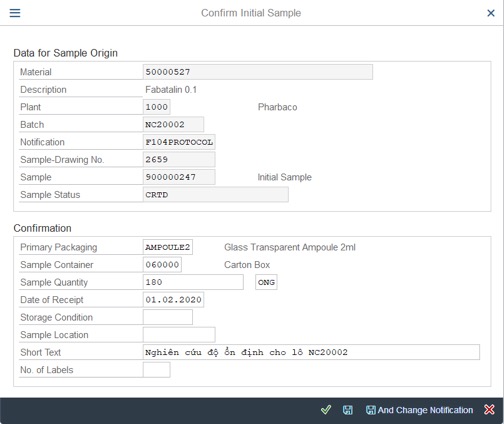
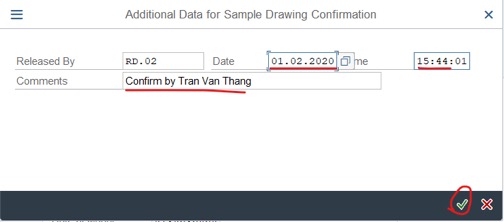
Step 2: Confirm sample: Edite Date, Time and add comment >> Click on V icon
Guidance on Pharmaceutical Stability Study with SAP S/4HANA Part 2/2

https://www.facebook.com/pharmalabs.rd
https://www.youtube.com/channel/UCzDND2lXy7whjG4UVauDvBA